Ion-exchange Chromatography
Ion exchange chromatography is a chromatographic technique that uses ion exchangers to have different affinities (electrostatic attraction) for various ions to be separated for separation purposes. The stationary phase of ion exchange chromatography is an ion exchanger, and the mobile phase is an electrolyte solution having a certain pH and a certain ionic strength.
The ion exchanger is an insoluble polymer compound having an acidic or basic group, and these charged acidic or basic groups are covalently bonded to the parent thereof, and the cation or anion attracted by these groups can be combined with the cation in the aqueous solution. Or the anion undergoes a reversible exchange. Thus ion exchangers are divided into two broad categories based on the nature of the exchangeable ions: cation exchangers and anion exchangers (Figure 3-3).
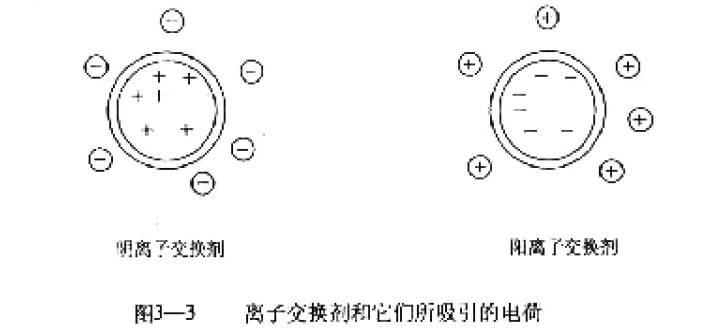
According to the chemical nature of the ion exchanger, it can be classified into a variety of ion exchange resins, ion exchange cellulose, and ion exchange dextran.
The ion exchange resin is a synthetic polymer compound, and the ion exchange resin used in the biochemical experiment is mostly a crosslinked polystyrene derivative. Ion exchange resins are mostly used for sample deionization, recovery of required ions and water from waste liquids, and the like. Because it denatures unstable biomacromolecules, it is not suitable for separation of biological samples. Shanghai Chuangsai Technology provides cation exchange resin, Amberlite®, IRP-69, 55464-99-8, product number: C16-A11394-100g, price 295 yuan.
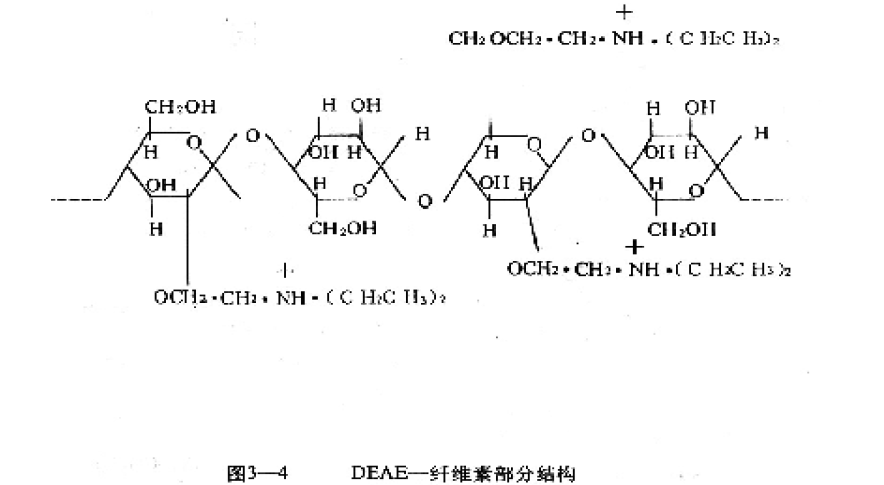
Ion exchanged cellulose (Figures 3-4) can be used for the separation of biological macromolecules. The shortcomings are irregular molecular morphology, non-uniform pores, and insufficient satisfaction with very demanding tests.
Preferred ion exchangers are ion exchange dextran gels and ion exchange agarose gels. They have the advantages of neat particles, uniform pore size, and often good separation results. Shanghai Chuangsai Technology provides anion exchange resin, Amberlite®, IRA402 chloride form, chlorine type, 52439-77-7, product number: C16-A11188-250g, price 195 yuan.
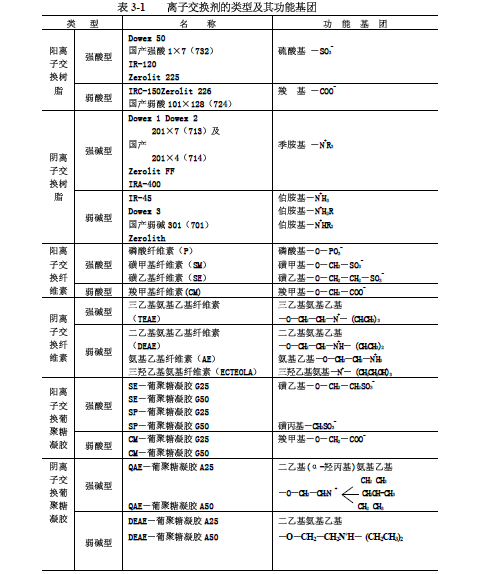
According to the difference of acidic and basic functional groups and the dissociation ability of various ion exchangers, various exchangers can be further divided into four types: strong acid type, weak acid type, strong alkali type and weak base type. Listed in Table 3-1.
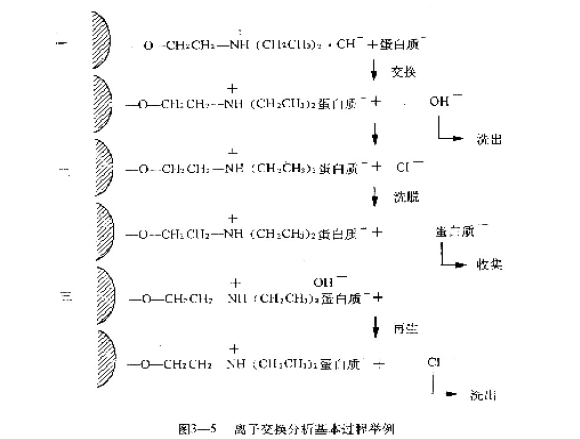
The basic process of ion exchange chromatography is: after the ion exchanger is properly treated and packed, it should be treated with acid or alkali (depending on the buffer of a certain pH as the case may be) to make the ion exchanger become the corresponding ionic type ( The cation exchanger is negatively charged and attracts the opposite ion H+. The anion exchanger is positively charged and attracts the opposite ion OH-. After the sample is added, the sample is exchanged with the opposite ion (H+ or OH-) attracted by the exchanger, and the sample is to be treated. The separated material is adsorbed onto the ion exchanger by covalent bonds (Fig. 3-5), and then sufficiently washed with a solution (such as a starting buffer) that does not substantially change the affinity of the exchanger to the ion of the sample, so that it is not adsorbed. The material is washed out. There are two methods for eluting the substance to be separated, one is to make the electrolyte concentration gradient, that is, the ionic strength gradient. By continuously increasing the ionic strength, the substance adsorbed to the exchanger is constantly competitive according to the magnitude of its electrostatic attraction. The second step is to make a pH gradient, which affects the ionization ability of the sample, and also reduces the affinity of the ion to the sample. When the pH gradient is close to the isoelectric point of each sample ion, Sub was eluted. In practice, the ionic strength gradient and a pH gradient may be continuous (called continuous gradient elution), or may be discontinuous (called elution phase).
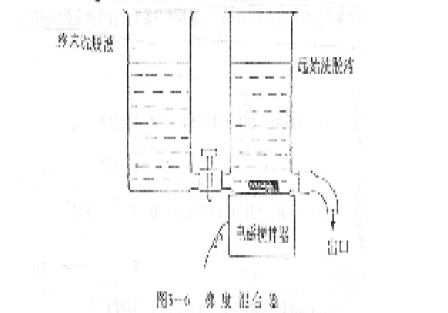
In general, the separation effect of the former is better than that of the latter, and the gradient elution requires a gradient mixer to produce an ionic strength gradient or a pH gradient. Figure 3-6 shows the simplest type of gradient mixer. It consists of two containers. The two containers are connected by a connecting pipe. The container connected to the outlet is equipped with a stirring device, which contains the initial eluent. The eluent represents the ionic strength (or initial pH) at which the elution begins; the other vessel contains the final eluent, which represents the last ionic strength (or final pH) of the elution. During the elution process, as the final eluent continuously enters the initial eluent and is continuously stirred evenly, the eluent component that flows out continuously evolves from the initial state to the terminal state to form a continuous gradient change. .
Shanghai Chuangsai Technology has excellent performance, interleukin cytokines, fetal bovine serum, electrophoresis equipment scientific instruments, raw material drug standards, chemical reagents, cell culture consumables, Shanghai Chuangsai, mass products special promotions, welcome to inquire!
3 Tier Aluminum Dish Rack
3 Tier Aluminum Dish Rack,Aluminum 3 Tier Dish Drainer,3 Layer Rose Aluminum Dish Rack,3 Layer Rose Gold Dish Racks
Jiangmen Xinhui Siqian Xiangyi Metalwork Factory , https://www.xydryingrack.com